The Boiling Points of Hydrogen Chloride and Hydrogen Bromide
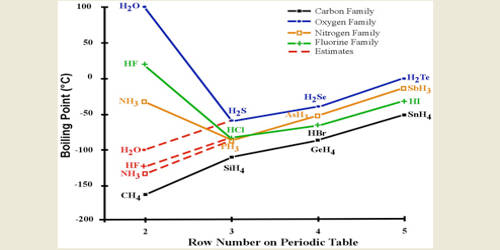
The boiling point of hydrogen flouride HF is the highest at 195oC. 70 C at 592 atm.
Why Melting And Boiling Points Of Hydrogen Fluoride Is Higher Than Hcl Hbr And Hi Qs Study
1333 K Boiling point 63 C 145 F.
. Arrange the following in order of increasing boiling point. Hydrogen iodide hydrogen bromide hydrogen chloride hydrogen flouride. Specific heat calg C.
The other halogens being less electronegative than Fluorine would therefore not form hydrogen bonds to a degree that fluorine does. Boiling point is determined by the energy required to break these intermolecular forces. Boiling point of Hydrogen Sulfide-60.
Hydrogen iodide hydrogen fluoride hydrogen bromide and hydrogen chloride. From these three compounds ethanol has the highest boiling point. Boiling point of Hydrogen-253 C.
The boiling point of hydrogen bromide HBr -6638oC and the boiling point of hydrogen chloride HCl is. H 2 SeO 4. H 3 PO 4.
Data pageExternal MSDS GHS pictograms GHS Signal word Warning GHS hazard statements H225 H304 H319 GHS. 10 rows Boiling point -121F Molecular weight 365 Freezing pointmelting point-174F. 0152 at -91 C solid.
H 2 SO 4. Heat of fusion at melting point. Boiling point of Hydrogen-253.
Arrange the following in order of increasing boiling point. Given this enhanced intermolecular interaction HI should have a higher boiling pointmelting point than HCl. Now we try to understand what are the reasons for those different boling points.
Whereas hydrogen chloride boils at -8005 degrees Celcius. Hydrogen iodide hydrogen fluoride hydrogen bromide and hydrogen chloride. But hang on HF has a boiling point of 195 C which is the highest of the hydrogen halides.
Appearance Colorless liquid Density 0673 gcm3 Melting point 1398 C 2196 F. Hydrogen bromide and hydrogen chloride are both simple molecules. 36 C at 300 atm.
They have London forces between molecules. Boiling point of Indium. Explain why the boiling point of hydrogen bromide lies between those of hydrogen chloride and hydrogen iodide FIRST i thought polar-molecules but they all are then I thought electronegativity but that would contradict the boiling points so.
You will have to look up the physical constants here is a start. It is essential that you highlight that these interactions induced dipole-dipole are BETWEEN the molecules rather than WITHIN them. 0085 at 27 C gas.
Boiling point -882F Molecular weight 8092 Freezing pointmelting point-124F. Boiling point of Hydrogen Chloride-817. 20 atm Flash point.
Therefore stronger intermolecular forces. 12 C at 171 atm. Oxygen O 2 hydrogen bromide HBr and ethanol C 2 H 5 OH have boiling points of -183 0 C -66 0 C and 7837 0 C respectively.
Saturated solution contains 6885 HBr at 0 C and 66 at 25 C. Explain why the heat of vaporization is typically significantly greater than the heat of fusion for any given substance. -48 C at 110 atm.
Which of the following contains the most polar bonds. Boiling point C hydrogen bromide. Hence the intermolecular force between the molecules of HF compound increases which causes higher melting and boiling points.
336 K Solubility in water Insoluble Viscosity 051 cP 051 mPas at 28C Hazards Safety data sheet See. Hydrogen iodide hydrogen bromide hydrogen chloride hydrogen flouride.

The Boiling Points Of Hf Hcl Hbr And Hi Follow The Order

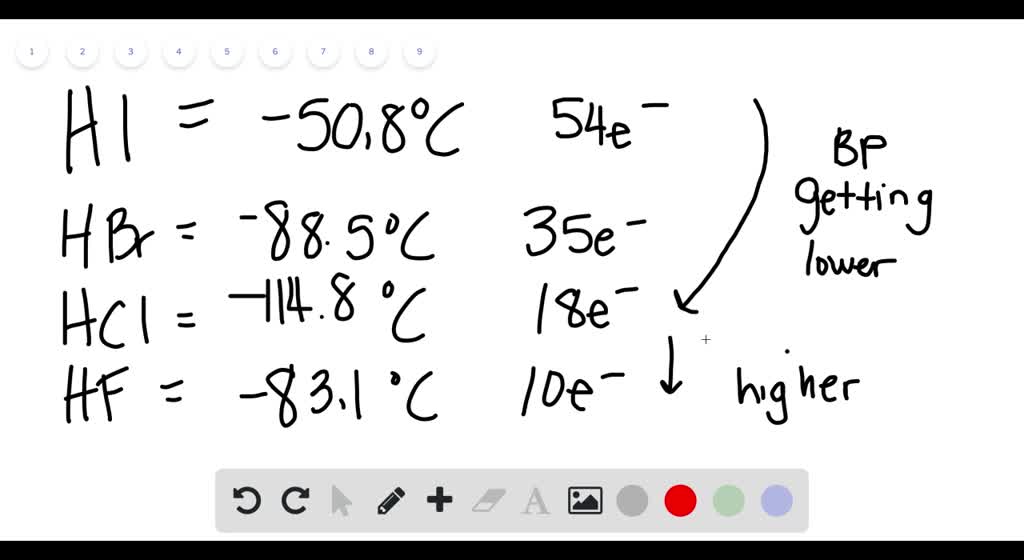
Solved Explain The Observed Trend In The Melting Points Of The Hydrogen Halides Hi 50 8 C Hbr 88 5 C Hcl 114 8 C Hf 83 1 C
Out Of Hf Hcl Hbr And Hi Which Has The Lowest And Highest Boiling Point And Why Quora
No comments for "The Boiling Points of Hydrogen Chloride and Hydrogen Bromide"
Post a Comment